
Akoya CODEX Multiplex Imager
The CODEX (Co-Detection by Indexing) technology combines the advantages of single-cell biology and histology. The CVR CODEX is combined with a Keyence BZ-X810 fluorescence microscope enabling highly multiplexed (40+) biomarker analysis in both fresh-frozen and formalin-fixed paraffin-embedded tissue sections. The combination of high-resolution imaging and high-parameter detection reveals different cell phenotypes, and how they interact and organize across the entire tissue landscape to impact disease pathology and progression.


Nexcelom Celigo Imaging Cell Cytometer
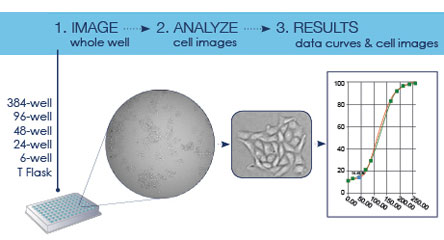
The Celigo is a bench-top, micro-well plate based, brightfield and fluorescent imaging system. It provides high speed, fully automated imaging, segmentation and quantification of suspension or adherence cells within 6, 12, 24, 48, 96, 384 or 1536-well plastic plates. Its proprietary optics and scanning system enables fast imaging of entire wells while maintaining consistent illumination and contrast out to the well edge, for accurate identification of all cells within each well. In 2022 a server and satellite workstation have also been added, expanding the capacity of the Celigo database and introducing an extra workstation for image processing.

Thermo Fisher EVOS M5000 Imager
The EVOS M5000 microscope is very easy to use and is the CVRs workhorse for routine fluorescent cell imaging. It has a high-resolution CMOS monochrome camera and the ability to capture three fluorescence channels plus transmitted light. Our EVOS M5000s are fitted with DAPI, GFP and mCherry LED illumination.
Thermo Fisher EVOS M7000 imager
The EVOS M7000 microscope is a fully automated, no-personal-contact imaging system. It incorporates both monochrome and colour high-resolution CMOS cameras for the best of both fluorescent and colorimetric imaging. The M7000 can scan multiwell plates automatically and features speedy autofocus, image acquisition, and large data processing.

Leica Aperio Versa 8 Slide Scanner
The Aperio allows automation of image acquisition from whole slides using brightfield or
Fluorescence modes. The stage holds 8 slides.
The dichroic filters available are:
- DAPI – excitation = 350/50, emission = 460/50
- Green - excitation = 485/25, emission = 525/30
- Orange - excitation = 546/22, emission = 590/33
- Red - excitation = 580/25, emission = 625/30
There are 5 (dry) objective lenses fitted, ranging from 1.25x to 40x.
Images are acquired using Aperio Versa software, with Imagescope software available for image viewing and editing. A companion image processing workstation also has Imagescope installed, with algorithms for detection of nuclei, cytoplasm, membranes and fluorescence.

Leica DMi8 Fluorescence Microscope
The Leica DMi8 is fitted with 3 long working distance objectives for use with plastic/glass media (10x,20x,40x) and a short working distance objective lens (100x/1.32,oil) for use with glass. The filter cubes currently installed are suitable for DAPI/FITC/rhodamine/mCherry/Alexa 647/ and spectrally similar fluorophores.

ONI Nanoimager
The Nanoimager is the CVR’s newest imager. It is a compact super-resolution imager that incorporates PALM, dSTORM, smFRET and SPT with built-in analysis software and is particularly useful for:
- dSTORM/PAL: Get up to 10 times better resolution than widefield microscopt. Resolving structures down to 20nm,
- Single-particle tracking (SPT): Follow particles on the nanometer scale simultaneously in two channels.
- Single-molecule FRET (smFRET): A real-time nanoscale ruler operating on a 2-10 nm range.

Zeiss Z.1 Lightsheet Microscope
The CVR’s Zeiss Z.1 lightsheet microscope is the only Zeiss Z.1 lightsheet microscope installed in Scotland. It provides flexibility in the CVR’s capacity for deep imaging. It can gently and quickly image large specimens, fixed (cleared or aqueous) or live, detailing dynamic biological processes and providing context to higher resolution imaging modalities in the CVR such as super-resolution light microscopy and cryo-electron microscopy.

Zeiss LSM 710 Meta Confocal Microscope
Purchased in 2012, the Zeiss LSM 710 meta utilises Zen 2011(black) software. The microscope’s spectral detector, which can be used to unmix up to 10 (spectrally similar) fluorophores simultaneously and remove autofluorescence, is also ideal for FRET and FRAP. Both scan speed and detector sensitivity are significantly enhanced compared to the CVR’s older LSM 510 meta system. Extra features include the functionality to create image montages, accurately identify colocalisation and enhance low signal in samples with a large range of signal intensities using the software’s High Dynamic Range module.
The microscope is fitted with the following hardware:
|
Objective lenses |
Laser lines |
Detectors |
|
10x (dry) – DIC ready |
405nm (eg DAPI) |
2 x PMTs |
|
20x (dry) – DIC ready |
458nm (eg CFP) |
1 x spectral detector |
|
40x (oil) – DIC ready |
488nm (eg GFP) |
|
|
63x (oil) – DIC ready |
514nm (eg YFP) |
|
|
100x (oil) – NOT DIC ready |
561nm (eg Alexa 568) |
|
|
|
633nm (eg Alexa 633) |
|

Zeiss LSM 880 Confocal Microscope with Airyscan FAST
Purchased in 2015, the Zeiss LSM 880 utilises Zen 2 (black) software. The system is fitted with a choice of autofocus options and an incubator incorporating full CO2 and temperature control allowing for extended live-cell time-lapse imaging. Other hardware installed:
|
Objective lenses |
Laser lines |
Detectors |
|
10x (dry) – DIC ready |
405nm (eg DAPI) |
2 x PMTs |
|
20x (dry) – DIC ready |
458nm (eg CFP) |
1 x GaAsP |
|
40x (oil) – DIC ready |
488nm (eg GFP) |
1 x Airyscan/FAST |
|
63x (oil) – DIC ready |
514nm (eg YFP) |
|
|
|
561nm (eg Alexa 568) |
|
|
|
594nm (eg Alexa 594) |
|
|
|
633nm (eg Alexa 633) |
|
The quantum efficiency of the GaAsP and Airyscan detectors provides significant improvement in signal:noise in comparison to the CVR’s LSM 710, which has only PMT detectors. The LSM 880 also offers improved scan speed.
Incorporation of an Airyscan detector allows for super-resolution imaging, providing an improvement of up to 2x higher resolution (in x,y and z dimensions) compared to ‘standard’ confocal microscopy.
The addition of the Airyscan FAST module in 2021 has increased the speed of Airyscan acquisition 4-fold and provides an expanded range of image resolutions. The associated workstation also allows for synchronised Airyscan processing which further speeds up the image acquisition process.

